Diagram Rusting Of Iron Experiment
Diagram Rusting Of Iron Experiment. Come browse our large digital warehouse of free sample essays. The exposure of iron (or an alloy of iron) to oxygen in the presence of moisture leads to the formation of rust. The reaction needs a considerable long time to develop. The iron was in the filings, the oxygen came from the air, and of. 15.3.1 rusting of iron wire see diagram 3.3.1: Purchase 2 pounds of iron. Growth & development experiment sed 695b; The rusting of iron is speeded up if iron is in contact with less electropositive metals such as tin or copper. Rust is not a direct product between iron and oxygen but arises through a complex electrochemical process. There is very little if any rust on the steel.
The other examples of the formation of rust takes place in the presence of water and oxygen on iron or some of its alloys. Chemically, rust is a hydrated ferric oxide. When iron is exposed to oxygen in the air, a similar reaction occurs do you think the rusty chain and door handle in the following photos will be as strong and flexible as the following diagram shows a segment of galvanised steel, with a scratch in the protective coating. While playing in your building compound, you might have come across an iron barbed wire which has turned red. Come browse our large digital warehouse of free sample essays. Rusting rates of iron nails. Rusting of iron takes place when iron corrodes in the presence of water and oxygen.it is a redox reaction whereby oxygen acts as an oxidising agent when iron is in contact with less electropositive of metal or example lead, rusting of iron is faster. However, if the coatings are washed away or scratched.

Rusting rates of iron nails.
Given sufficient time, oxygen, and water, any iron mass will eventually convert entirely to rust and disintegrate. Chemically, rust is a hydrated ferric oxide. In this way, the iron nail in the first test tube is kept in dry air. There is very little if any rust on the steel. 1) what factors cause iron the rust. Rusting iron wire polish 0.4 g (about 130 cm long) of thin iron wire this suggests that when iron filings rust, oxygen is used. Rusting is the common term for corrosion of iron and its alloys, such as steel. Iron objects react with the oxygen present in the air and develop rust in a humid environment. The surface of iron at the middle of the water droplet serves as the anode, the electrode at which oxidation occurs. You would realise that these objects have turned reddish, unlike their original metallic colour. Iron is easily prone to rusting making its surface rough. Fill up test tubes as follows: Rust is a form of iron oxide. Rusting is a redox reaction whereby oxygen acts as the oxidising agent and iron acts as the reducing agent.
You would realise that these objects have turned reddish, unlike their original metallic colour. The exposure of iron (or an alloy of iron) to oxygen in the presence of moisture leads to the formation of rust. The experiment in the diagram shows that both oxygen and water are needed for rusting to happen. Surface rust is flaky and friable, and provides no protection to the. There is very little if any rust on the steel. Rust is a broad topic of discussion for science classrooms at all grade levels.
Experiment not set up long enough the iron has not had enough time to react with all oxygen.
Repeat the experiment with magnesium. The exposure of iron (or an alloy of iron) to oxygen in the presence of moisture leads to the formation of rust. Growth & development experiment sed 695b; Rust is not a direct product between iron and oxygen but arises through a complex electrochemical process. The iron was in the filings, the oxygen came from the air, and of. Connect the drdaq datalogger to the parallel port (or usb if you are using a usb to parallel. For more advanced experiments in the real world, i.e., where time and money are a concern, there are doe (design of. Rust is a broad topic of discussion for science classrooms at all grade levels. Fill up test tubes as follows: Experiment not set up long enough the iron has not had enough time to react with all oxygen. To get to the oxygen, however, these electrons rust appeared on the iron filings in jar 1 because all reactants were present: The iron atoms here lose. 15.3.0 rusting rusting is the corrosion of iron and iron based alloys, eg.
In the experiment below, the nail does not rust when air (containing oxygen) or water is not present There is very little if any rust on the steel. Given sufficient time, oxygen, and water, any iron mass will eventually convert entirely to rust and disintegrate. While playing in your building compound, you might have come across an iron barbed wire which has turned red. The iron construction structure is tied with a bag filled with magnesium or zinc powder ( or magnesium or zinc pieces ) to protect the iron from getting corroded. Rusting is the common term for corrosion of iron and its alloys, such as steel.

Basic physics behind rusting of iron.
When rusting occurs, iron atoms lose electrons to the oxygen atoms. You would realise that these objects have turned reddish, unlike their original metallic colour. To study the effect of metal coupling on rusting of iron. Rust is not a direct product between iron and oxygen but arises through a complex electrochemical process. The exposure of iron (or an alloy of iron) to oxygen in the presence of moisture leads to the formation of rust. However, if the coatings are washed away or scratched. To construct an experimental design diagram for an experiment with one independent variable knowing how to construct an experimental design diagram is of little use unless you can apply iron nail with aluminum 6 nails. The experiment in the diagram shows that both oxygen and water are needed for rusting to happen. The rusting of iron is speeded up if iron is in contact with less electropositive metals such as tin or copper. Rusting is the common term for corrosion of iron and its alloys, such as steel. During the rusting of iron, oxygen from the air reacts with it to form iron oxide and, at the same time this iron oxide combines with water to form hydrated the experiment should be set up as shown in figure 1. This reaction is not instantaneous, it generally proceeds over a considerably large time frame. 1) what factors cause iron the rust. Rusting is a redox reaction whereby oxygen acts as the oxidising agent and iron acts as the reducing agent. Test tube a is filled with boiled water and an oil layer so that there is no oxygen.
This reaction is not instantaneous, it generally proceeds over a considerably large time frame rusting of iron diagram. The iron construction structure is tied with a bag filled with magnesium or zinc powder ( or magnesium or zinc pieces ) to protect the iron from getting corroded.
 Source: chem.libretexts.org
Source: chem.libretexts.org Investigating the rusting (oxidation) of iron.
 Source: haygot.s3.amazonaws.com
Source: haygot.s3.amazonaws.com Rusting of iron takes place when iron corrodes in the presence of water and oxygen.it is a redox reaction whereby oxygen acts as an oxidising agent when iron is in contact with less electropositive of metal or example lead, rusting of iron is faster.
 Source: swh-826d.kxcdn.com
Source: swh-826d.kxcdn.com You might have also seen some old metallic object at home.
 Source: hi-static.z-dn.net
Source: hi-static.z-dn.net In this way, the iron nail in the first test tube is kept in dry air.
Conditions for rusting iron and steel rust when they come into contact with water and oxygen.
 Source: haygot.s3.amazonaws.com
Source: haygot.s3.amazonaws.com 1) what factors cause iron the rust.
 Source: image.slidesharecdn.com
Source: image.slidesharecdn.com The following experiment investigates the conditions necessary for rusting to occur.
 Source: www.docbrown.info
Source: www.docbrown.info Given sufficient time, oxygen, and water, any iron mass will eventually convert entirely to rust and disintegrate.
 Source: swh-826d.kxcdn.com
Source: swh-826d.kxcdn.com In the experiment below, the nail does not rust when air (containing oxygen) or water is not present
 Source: cdn1.byjus.com
Source: cdn1.byjus.com Rust is not a direct product between iron and oxygen but arises through a complex electrochemical process.
 Source: i.ytimg.com
Source: i.ytimg.com To get to the oxygen, however, these electrons rust appeared on the iron filings in jar 1 because all reactants were present:
 Source: funscience.in
Source: funscience.in 2)what factors prevent of inhibit rusting.
 Source: quizlet.com
Source: quizlet.com Purchase 2 pounds of iron.
 Source: image.slidesharecdn.com
Source: image.slidesharecdn.com Iron is easily prone to rusting making its surface rough.
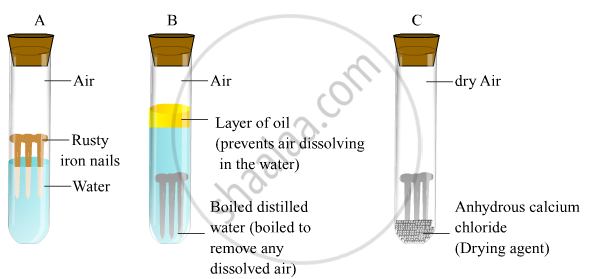
 Source: www.shaalaa.com
Source: www.shaalaa.com Rusting is a redox reaction whereby oxygen acts as the oxidising agent and iron acts as the reducing agent.
 Source: i1.wp.com
Source: i1.wp.com Rusting rates of iron nails.
 Source: www.coursehero.com
Source: www.coursehero.com While playing in your building compound, you might have come across an iron barbed wire which has turned red.
 Source: www.aplustopper.com
Source: www.aplustopper.com The anhydrous calcium chloride is added to absorb water or moisture from the damp air present in the test tube and make it dry.
 Source: revision.co.zw
Source: revision.co.zw Rusting is a redox reaction whereby oxygen acts as the oxidising agent and iron acts as the reducing agent.
 Source: i.ytimg.com
Source: i.ytimg.com Chemically, rust is a hydrated ferric oxide.
 Source: useruploads.socratic.org
Source: useruploads.socratic.org Chemically, rust is a hydrated ferric oxide.
 Source: 2.bp.blogspot.com
Source: 2.bp.blogspot.com The exposure of iron (or an alloy of iron) to oxygen in the presence of moisture leads to the formation of rust.
Detailed revision notes on the topic iron & rusting.
 Source: www.docbrown.info
Source: www.docbrown.info The iron construction structure is tied with a bag filled with magnesium or zinc powder ( or magnesium or zinc pieces ) to protect the iron from getting corroded.
 Source: hi-static.z-dn.net
Source: hi-static.z-dn.net Rusting rates of iron nails.
Magnesium or zinc, which is.
 Source: funscience.in
Source: funscience.in Connect the drdaq datalogger to the parallel port (or usb if you are using a usb to parallel.
 Source: image.slidesharecdn.com
Source: image.slidesharecdn.com The oxygen atoms bond with iron atoms
 Source: i.ytimg.com
Source: i.ytimg.com Purchase 2 pounds of iron.
 Source: corrosion-doctors.org
Source: corrosion-doctors.org To get to the oxygen, however, these electrons rust appeared on the iron filings in jar 1 because all reactants were present:
 Source: image.slidesharecdn.com
Source: image.slidesharecdn.com The iron nail in test tube a will be covered with a layer of rust after a few days.
 Source: ars.els-cdn.com
Source: ars.els-cdn.com Iron objects react with the oxygen present in the air and develop rust in a humid environment.
 Source: i.imgur.com
Source: i.imgur.com Rusting may be explained by an electrochemical mechanism.
 Source: revision.co.zw
Source: revision.co.zw In the experiment below, the nail does not rust when air (containing oxygen) or water is not present
 Source: image.slidesharecdn.com
Source: image.slidesharecdn.com Given sufficient time, oxygen, and water, any iron mass will eventually convert entirely to rust and disintegrate.
 Source: www.canada.ca
Source: www.canada.ca The reaction needs a considerable long time to develop.
 Source: swh-826d.kxcdn.com
Source: swh-826d.kxcdn.com When rusting occurs, iron atoms lose electrons to the oxygen atoms.
 Source: dryuc24b85zbr.cloudfront.net
Source: dryuc24b85zbr.cloudfront.net In the presence of moist air containing dissolved oxygen or carbon dioxide, the commercial iron behaves as if composed of small electrical.
 Source: ars.els-cdn.com
Source: ars.els-cdn.com Basic physics behind rusting of iron.
Posting Komentar untuk "Diagram Rusting Of Iron Experiment"